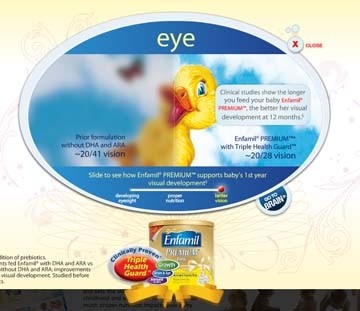
'Eye' or 'nay': For or against the DHA infant eye claim?

Here we detail the position of some of those groups lobbying the 736 Members of the European Parliament (MEPs) as they prepare to cast their votes in the Parliament's Strasbourg, France, base next week.
Baby Milk Action
"This is first time since the 2006 European Health and Nutrition Claims Regulations came into effect that MEPS have used their right to block a claim and the news was greeted with huge relief by thousands of health professionals and public health campaigners both in the EU and globally who have been fighting to protect parents rights to truly independent information about infant feeding.
"If the legislation controlling baby food marketing was more transparent and democratic, people would soon realise that such highly promotional claims on baby formulas and foods are totally inappropriate and harmful to child health. "
European Association of Craft, Small and Medium-Sized Enterprises
"This is a quite worrying development regarding EFSA and the European Parliament.
"A small group of MEPs, including Mrs Willmott (UK, S&D) tries to put into question the independent scientific findings of EFSA. This relates to only one claim dealing with omega-3/DHA for infant food. If this resolution of the EP would be positively voted next week, this would undermine the scientific independence of EFSA, which itself was demanded by the European Parliament.
"If a company has put forward all the necessary evidence to meet EFSA’s gold standard and has consequently received a positive scientific opinion on its health claim, it should not have to face a secondary review second-guessing EFSA’s judgment on the science.
"A further review would constitute an additional and unnecessary hurdle (risk) further discouraging investment and support from industry. Such additional hurdles and risks will result in a further economic downturn in Europe due to decreasing investment and innovation in the food and health sector."
Socialist MEP Glenis Willmott, Co-Chair of the EP Health Working Group
"The European Parliament delegated the power to make decisions about infant feeding to the Commission and a specialist committee, which meets behind closed doors. However MEPs have an important role to play in scrutinising these decisions as this claim shows.
"Independent studies say there is no proven link between artificially added DHA and eyesight, and some studies have found possible negative effects of DHA supplementation. As the scientific evidence is still inconclusive, we cannot allow parents to be misled. Babies' health is too important to be left in the hands of a multinational company's marketing department."
Global Organization for EPA and DHA Omega-3s
"The benefits of breast milk are undisputed, so the issue is not which is best, breast feeding or formula feeding; the issue is whether or not DHA‐supplemented formula provides an advantage over unsupplemented formula.
"EFSA has provided a positive opinionon the claim in question. Also, contrary to allegations that EFSA’s opinion conflicts with 'leading scientific opinion', this opinion is based on the totality of the available scientific evidence, including 43 relevant scientific publications of which 13 were randomized clinical trials.
"DHA is selectively concentrated and excreted as a natural constituent of human breast milk and as a result DHA is recognized in both the existing European Regulation on nutritional content of infant formula, and by the international Codex Alimentarius Commission’s Standard for Infant Formula.
"The inclusion of DHA in infant formula represents a technological improvement by the manufacturers to benefit the nutrition of infants."
The International Association for the Study of Obesity and the International Obesity TaskForce
"We are supporters of the International Code of Marketing of Breastmilk Substitutes and believe that breastfeeding is beneficial both for the infant and for the mother in the prevention of chronic disease. Factors which undermine breastfeeding include the use of health claims to promote infant formulae and follow-on milks."
United Nations Children's Fund (UNICEF)
"It is UNICEF’s view that a follow-on formula is as much a breastmilk substitute as infant formula. Indeed, follow-on formulas did not exist when the International Code of Marketing of Breastmilk Substitutes was adopted in 1981, and were developed by the baby food industry to try and get around the prohibition on promotion in the Code.
"There can be little doubt that the use of such health claims can mislead parents into thinking that the formulas are as good as, if not better than breastmilk. They are not informed that the claims of improved visual development are based on a comparison with non-fortified artificial formula, and not with breastmilk, which the evidence shows leads to optimal survival, growth and development.
"Most recently, in 2010 the World Health Assembly adopted WHA 63.23 calling on Member States 'to end inappropriate promotion of food for infants and young children and to ensure that nutrition and health claims shall not be permitted for foods for infants and young children, except where specifically provided for, in relevant Codex Alimentarius standards or national legislation'.
"The EU Commission 'Infant and young child feeding: Standard recommendations for the European Union' and 'Protection, promotion and support of breastfeeding in Europe: a blueprint for action' both follow the WHA recommendation that, as a public health policy, breastfeeding should continue for two years or beyond. It would thus seem that allowing health claims in relation to breastmilk substitutes, including follow-on formula, would undermine this policy."
"On the basis, therefore, of these observations, UNICEF would be supportive of the proposed resolution opposing health claims."